
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
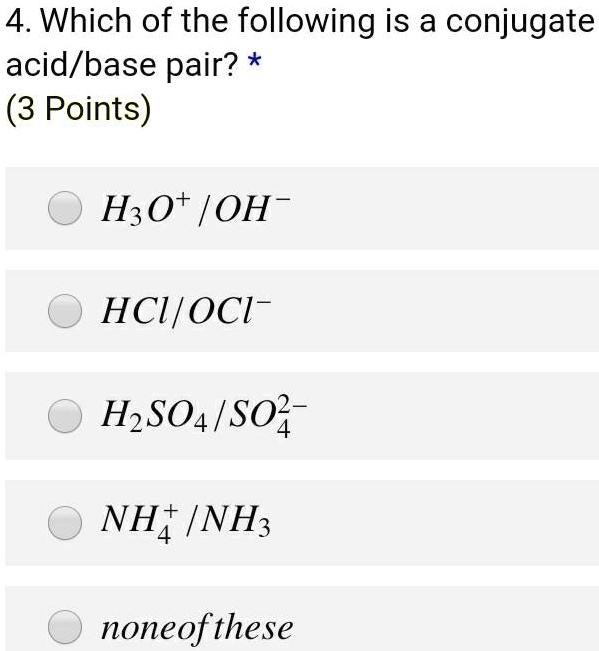
SOLVED: 4. Which of the following is a conjugate acid/base pair? (3 Points) H3O+ /OH HCl/OCL - HzSO4/S04 NHt /NH3 noneofthese

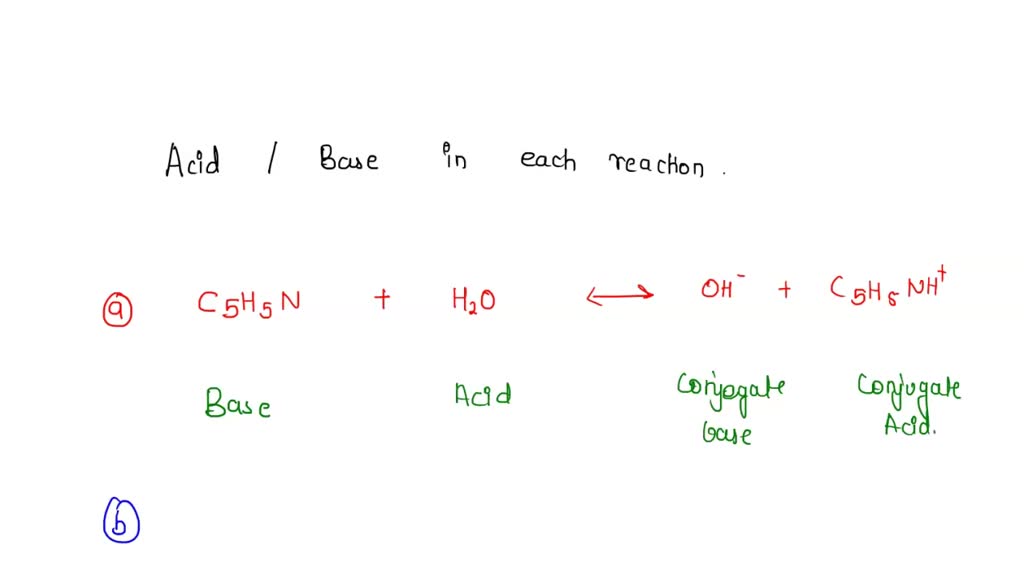
Why is oh- acid and h3o+ base H20(/) + H20(/) Acid Base H30+(aq) + OH-caq) Conjugate Conjugate base acid - Chemistry - Equilibrium - 13361499 | Meritnation.com
![Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube](https://i.ytimg.com/vi/UiK37I159fc/maxresdefault.jpg)
![16.6 Finding the [H3O+] & pH of Strong & Weak Acid Solutions - YouTube 16.6 Finding the [H3O+] & pH of Strong & Weak Acid Solutions - YouTube](https://i.ytimg.com/vi/UyBvt7-PDi0/hqdefault.jpg)
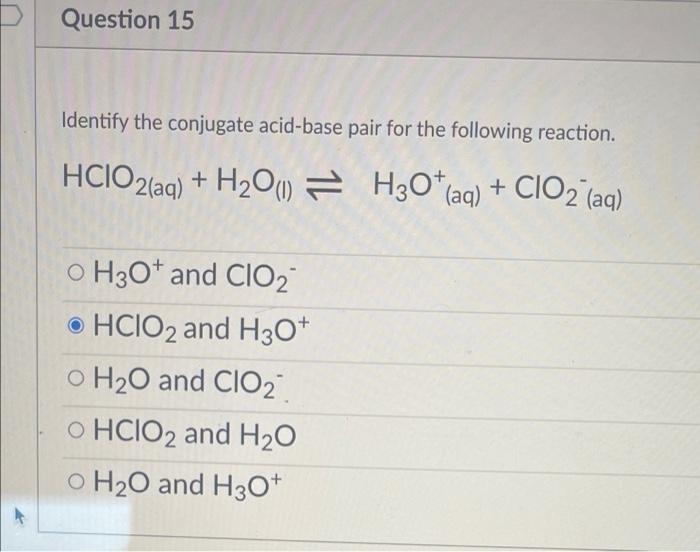
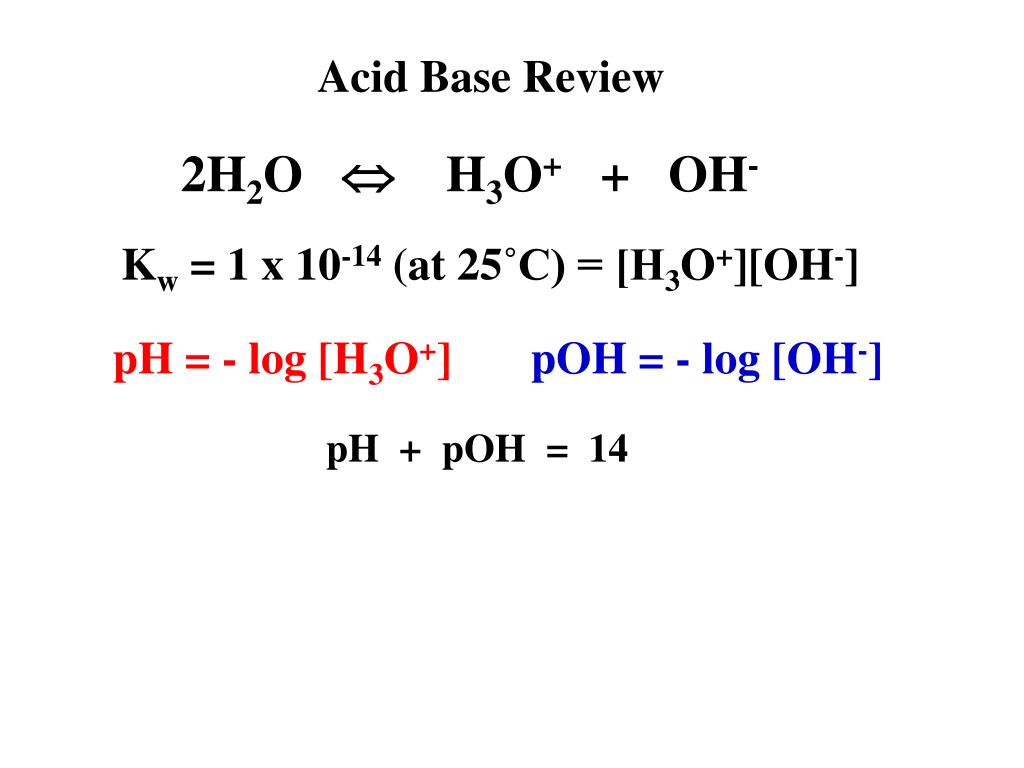
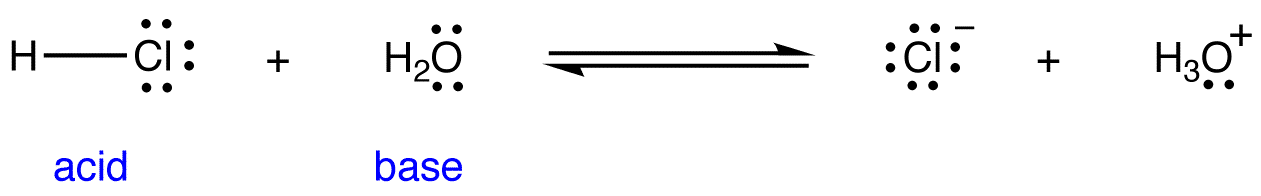
/chapter3/pages33and34/page33and34_files/ptsoh.png)
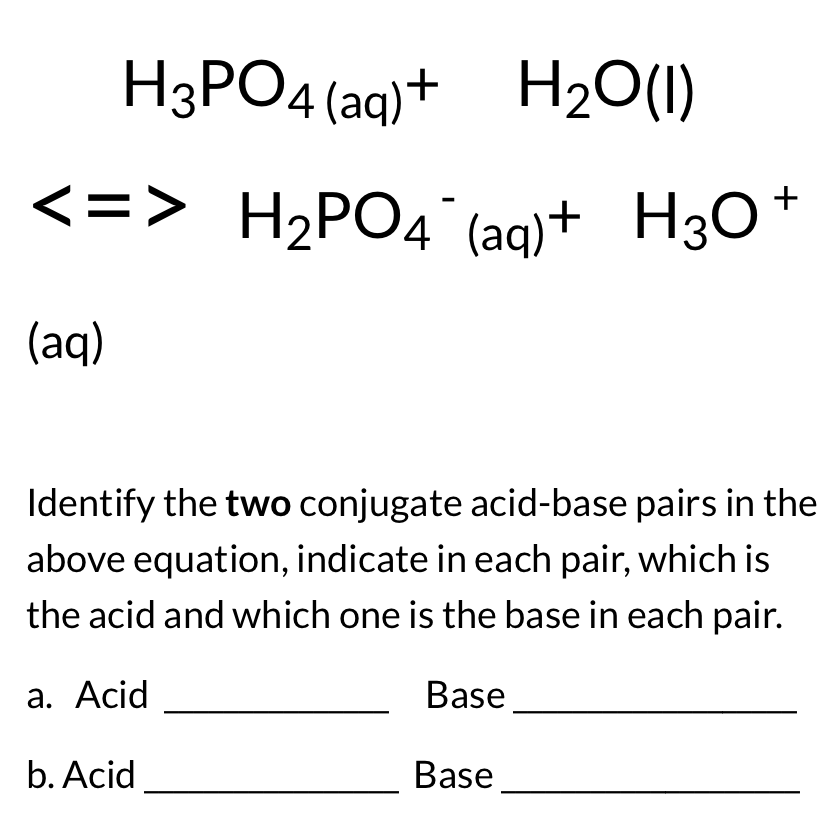
![Solved [H3O+] [OH-] pH pОН Acidic, Base or Neutral? | Chegg.com Solved [H3O+] [OH-] pH pОН Acidic, Base or Neutral? | Chegg.com](https://media.cheggcdn.com/media/f34/f34e841e-96b5-4eae-8369-3ddf8b4861c0/phpT8rw9L)

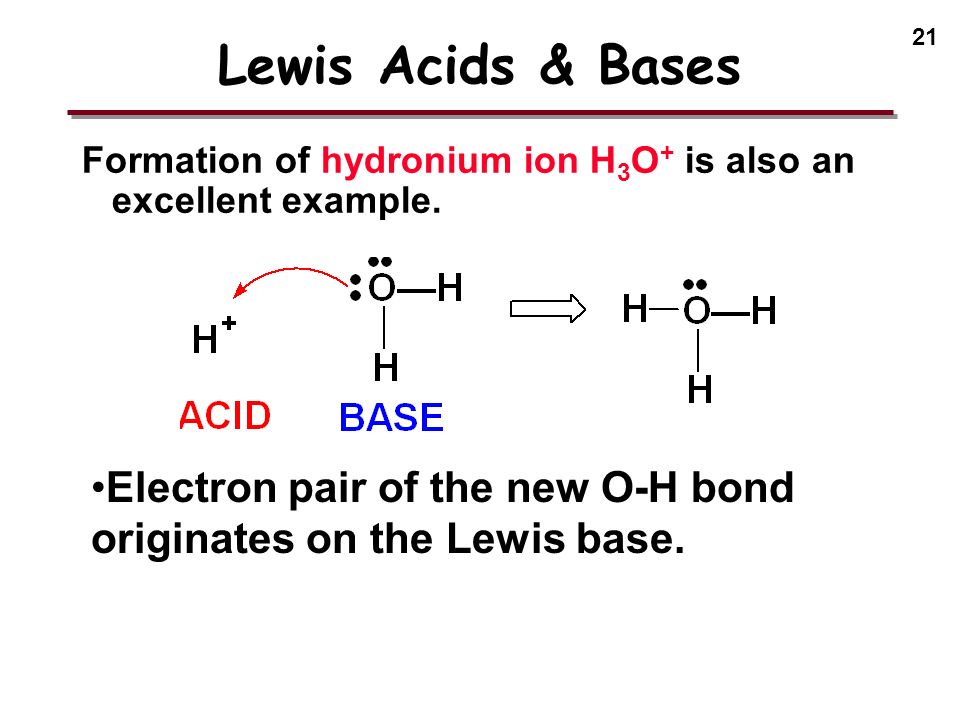
/chapter3/pages33and34/page33and34_files/aqh3o.png)

![16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions - Chemistry LibreTexts 16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions - Chemistry LibreTexts](https://i.ytimg.com/vi/y7DTjgrcP-0/maxresdefault.jpg)
![Acidic: [H3O+] > [OH-] Alkaline (basic): [OH-] > [H3O+] - ppt download Acidic: [H3O+] > [OH-] Alkaline (basic): [OH-] > [H3O+] - ppt download](https://slideplayer.com/slide/13521924/82/images/2/Acidic%3A+%5BH3O%2B%5D+%3E+%5BOH-%5D+Alkaline+%28basic%29%3A+%5BOH-%5D+%3E+%5BH3O%2B%5D.jpg)

![Get Answer) - Complete The Following Table: [H3O+] [OH-] PH POH Acidic, Basic,...| Transtutors Get Answer) - Complete The Following Table: [H3O+] [OH-] PH POH Acidic, Basic,...| Transtutors](https://files.transtutors.com/book/qimg/45efeeca-22db-48df-9654-ed26b44a10ab.png)